Efficacy
Clinically proven to control clonic and tonic-clonic seizures
DIACOMIT established efficacy in two randomized, double-blind, placebo-controlled Phase 3 clinical trials.1,2 Pivotal trials, STICLO France (n=41) and STICLO Italy (n=23) had identical protocols, enabling the pooling of data. Both were divided into a baseline, comparison, and 30-day open-label (OLE) extension period.1,3,4
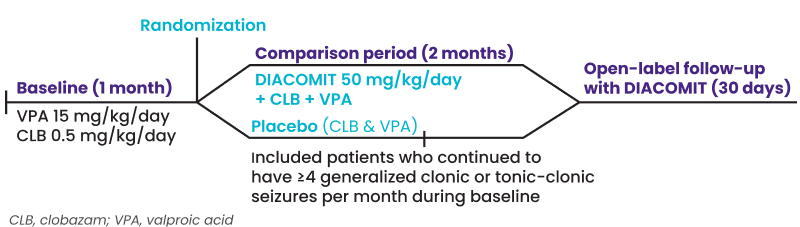
Inclusion Criteria
Age 3 to less than 18 years old
Confirmed Dravet diagnosis
Inadequately controlled seizures on CLB & VPA
4+ generalized clonic or tonic-clonic seizures monthly
Definitions
- The primary endpoint was responder rate. Response was defined as a ≥ 50% decrease in generalized clonic or tonic-clonic seizure frequency during the second month of treatment compared with baseline
- “Seizure-free” was defined as experiencing no generalized clonic or tonic-clonic seizures for the duration of the study
- Baseline was one month on clobazam and valproate before patients entered the double-blind trial for two months on either DIACOMIT (50 mg/kg/day) or placebo
- Statistical significance was measured with a Fisher Exact Test, generating a P value of <0.0001
Pivotal Trials Pooled Results:
74% of Patients Achieved the Primary Endpoint1,2
Double-blind period (2 months)
Primary Endpoint: ≥ 50% Reduction in Seizure Frequency
39% seizure-free
Nearly 39% of patients on DIACOMIT experienced no generalized clonic or tonic-clonic seizures compared with 0% on placebo during the two-month period.1,2
Open-Label Extension Pooled Results:
~88% of Patients Who Switched to DIACOMIT Achieved Meaningful Seizure Reduction4
Open-label extension (1 month)
Primary Endpoint: ≥ 50% Reduction in Seizure Frequency
64% remained seizure-free
In the DIACOMIT group, 64% of those who were seizure free at the end of the double-blind period remained so during the open-label extension.4
In the OLE, no change in efficacy was observed for patients already on DIACOMIT.4
Substantial Median Decreases in Seizure Frequency
Double-blind period
DIACOMIT reduced clonic or tonic-clonic seizures by 84% compared with a 5.8% reduction on placebo after two months on treatment.2
Open-label extension
Patients who switched to DIACOMIT from placebo saw an 85.2% decrease in seizures compared to baseline after one month.4
Demonstrated Efficacy for Patients in the US*
In a retrospective study, the majority of patients experienced reduction in frequency of prolonged seizures, rescue medication use, and emergency room or hospital visits with stiripentol.5 The study included records from thirteen physicians using stiripentol to treat two or more patients (n=82) with Dravet between March 2005 and 2012.5
Patients on stiripentol and clobazam (n=35) reached an 80% reduction in seizure frequency.5
Adverse events were reported in 31 patients (n=82), which were most commonly somnolence and reduced appetite.
*This study had limitations, including that retrospective chart review is less accurate than prospective counts; patients were on varied other comedications, and their dose changes were not formally assessed; and adverse events were based on chart review, which may have limited reporting of milder side effects.
References
1. DIACOMIT® [prescribing information]. Beauvais, France: Biocodex, Inc.; July 2022. 2. U.S. Food and Drug Administration. CDER Clinical Review. August 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/206709Orig1s000,207223Orig1s000MedR.pdf. Accessed May 12, 2020. 3. Chiron C, Marchand MC, Tran A, et al; for the STICLO study group. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome dedicated trial. Lancet. 2000;356(9242):1638-1642. 4. Guerrini R, Chancharme L, Serraz B, Chiron C. Additional results from two randomized, placebo-controlled trials of stiripentol in Dravet syndrome highlight a rapid antiseizure efficacy with longer seizure-free periods. Neurol Ther. 2024;13:869-884. 5. Wirrell E, et al. Stiripentol in Dravet syndrome: Results of a retrospective U.S. study. Epilepsia. 2013;54(9):1595-1604.